Stable isotope analysis of non-exchangeable hydrogen in carbohydrates derivatised with N-methyl-bis-trifluoroacetamide by gas chromatography – Chromium silver reduction/High temperature Conversion-isotope ratio mass spectrometry (GC-CrAg/ HTC-IRMS)
Aiman Abrahim, Andrew Cannavan, Simon D. Kelly
Food and Environmental Protection Laboratory, Joint FAO/IAEA Division of Nuclear Applications in Food and Agriculture, Department of Nuclear Sciences and Applications, International Atomic Energy Agency, Vienna International Centre, PO Box 100, 1400 Vienna, Austria
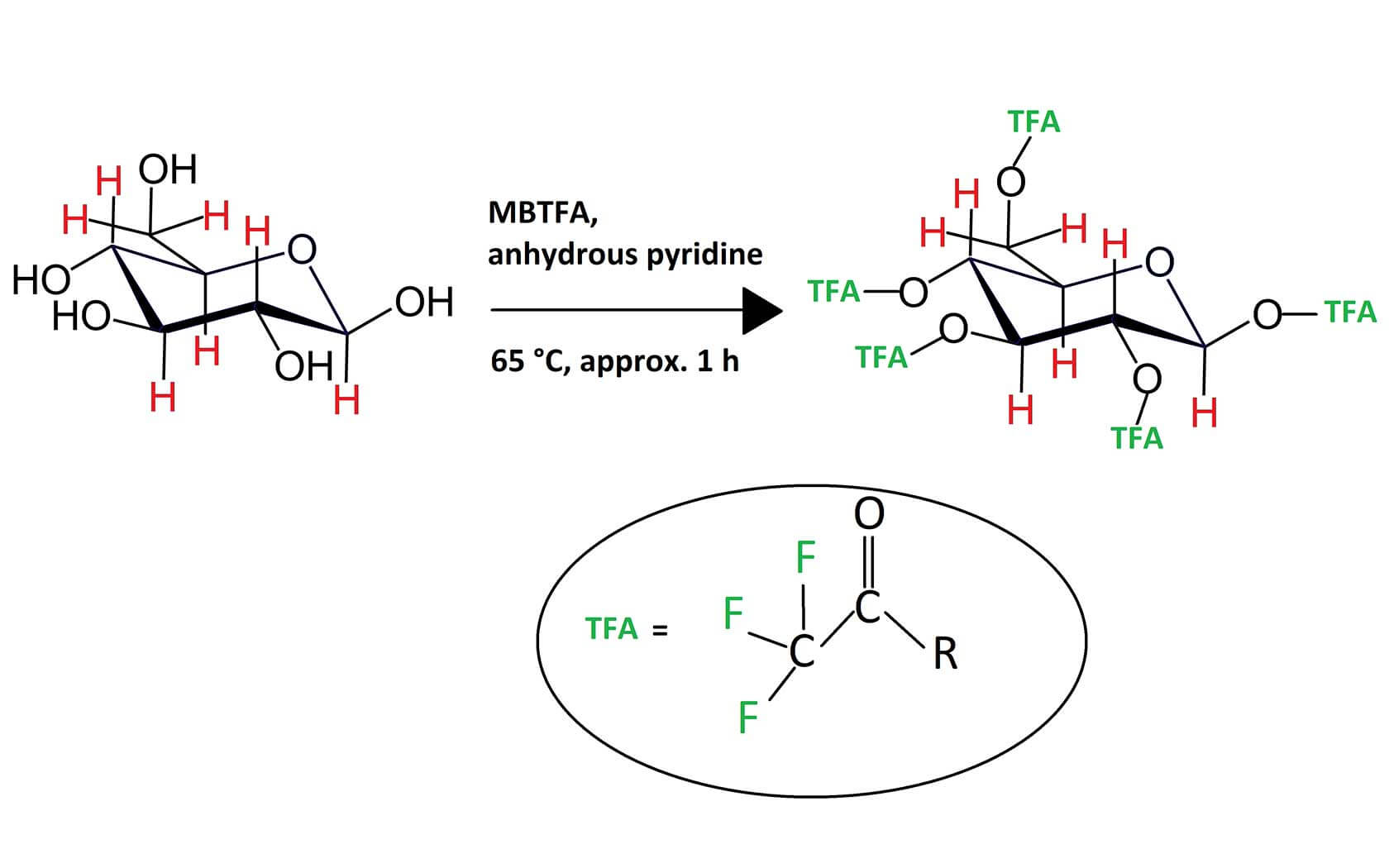
The measurement of δ2H values of the carbon-bound non-exchangeable (CBNE) hydrogen atoms in sugars, after derivatisation of the hydroxyl-hydrogen with a nitro-group through nitric acid esterification, can be used to authenticate fruit juices and wine. This approach was not used extensively due to time-consuming and potentially hazardous preparative methods or leading to potential fractionation of the isotopomers of the target compound. Recently, a simple and effective procedure has been developed to enable such investigation. A derivatizing agent N-methyl-bis-trifluoroacetamide substitutes the exchangeable hydroxyl-hydrogens with trifluoroacetate derivatives which are sufficiently volatile to be separated and measured by a gas chromatograph (GC) coupled to an isotope ratio mass spectrometer (IRMS). The conversion of the derivatized sugars into the measuring gas is achieved using an Elementar GC5 GC-IRMS system configured with a high temperature chromium-silver reactor that retains carbon, oxygen and fluorine whilst releasing hydrogen gas for stable isotope analysis.
Besides the advantages of using sugar-TFA derivatives to measure the stable isotopic composition of CBNE hydrogen atoms, there are some analytical challenges due to the formation of hydrogen fluoride (HF). Under typical high temperature conversion conditions around 1400 °C in an open ceramic tube, coated with carbon the formation of HF effects the repeatability of δ2H measurements. This issue can be avoided by utilizing Elementar’s ChromHD technology where organic compounds undergo on-line reduction over chromium metal (Cr/HTC) to H2 at 1200 °C. This technique retains the fluorine as chromium fluoride in the cooler part of the reactor, with no consequent formation of HF. Reagent grade monosaccharides and disaccharide fructose, glucose and sucrose were analyzed over 3 days to test repeatability and the precision of the δ2H analysis was ≤ 2.7 ‰. Sugars from various fruit juice and honey were then measured to demonstrate the feasibility of using this technique.
This single- step, rapid carbohydrate hydroxyl-hydrogen derivatisation process with MBTFA to produce volatile sugar derivatives proved to be reliable and applicable to real food samples. The precision of the data provided by the Elementar BiovisION system and the isotope fractionation-free conversion of the GC samples thanks to the GC5 furnace, provide a powerful tool in the food authenticity and traceability field. Furthermore, by using this method for examination of δ2H values of the CBNE H of glucose derived from the hydrolysis of complex polysaccharides, such as starch, glycogen and cellulose, widen its range of applications to other fields such as biomedical, ecology, environmental and forensic science.
Please find the full paper here: https://doi.org/10.1016/j.foodchem.2020.126413