There are two frequently used reference methods for determining the nitrogen and protein content in a sample, which have coexisted for over 140 years: Nitrogen determination according to Kjeldahl and nitrogen determination according to Dumas.
Nitrogen determination according to Kjeldahl
The Kjeldahl method was developed by Johan Kjeldahl at Carlsberg Laboratories in 1883, about 50 years after Jean-Baptiste Dumas presented his method for the determination of nitrogen. Since it was difficult to reproduce the conditions of the Dumas method, the wet-chemical Kjeldahl method was preferred for a long time and has established itself as the classic method for the determination of nitrogen and protein.
In the Kjeldahl nitrogen method, the sample is chemically digested with concentrated sulfuric acid and a catalyst at 420 °C for 90 minutes, converting the nitrogen in the sample to ammonium sulfate. Sodium hydroxide is then added to the ammonium solution. The ammonia that is released is then distilled and transferred to a boric acid solution. This is followed by titration with sulfuric or hydrochloric acid for quantitative determination of nitrogen. The protein content can then be calculated using a specific conversion factor.
Nitrogen determination according to Dumas
Jean-Baptiste Dumas presented his method for nitrogen determination in 1831. Although the Dumas method, based on the high-temperature combustion process, is the older method for determining nitrogen and protein content, it has only become established as the reference method for nitrogen determination in the last few decades. The reason: The development of modern analyzers made it possible to achieve consistent analysis conditions and reproducible results. Since the introduction of stricter environmental regulations in the 1990s, the environmentally friendly Dumas method has gained in popularity and increasingly replaced the classic Kjeldahl method, which relies on the use of harmful chemicals.
The Dumas method of nitrogen determination is based on combustion of the sample at a minimum of 950 °C in an oxygen-enriched environment. During combustion, all the nitrogen in the sample is converted to nitrogen oxides, which are then reduced to nitrogen and quantified using a thermal conductivity detector. The protein content can then be calculated using a specific conversion factor.
Comparison: Kjeldahl Method vs. Dumas Method
The following section compares the two methods in terms of analysis time, cost per analysis and safety.
Analysis time & personnel deployment
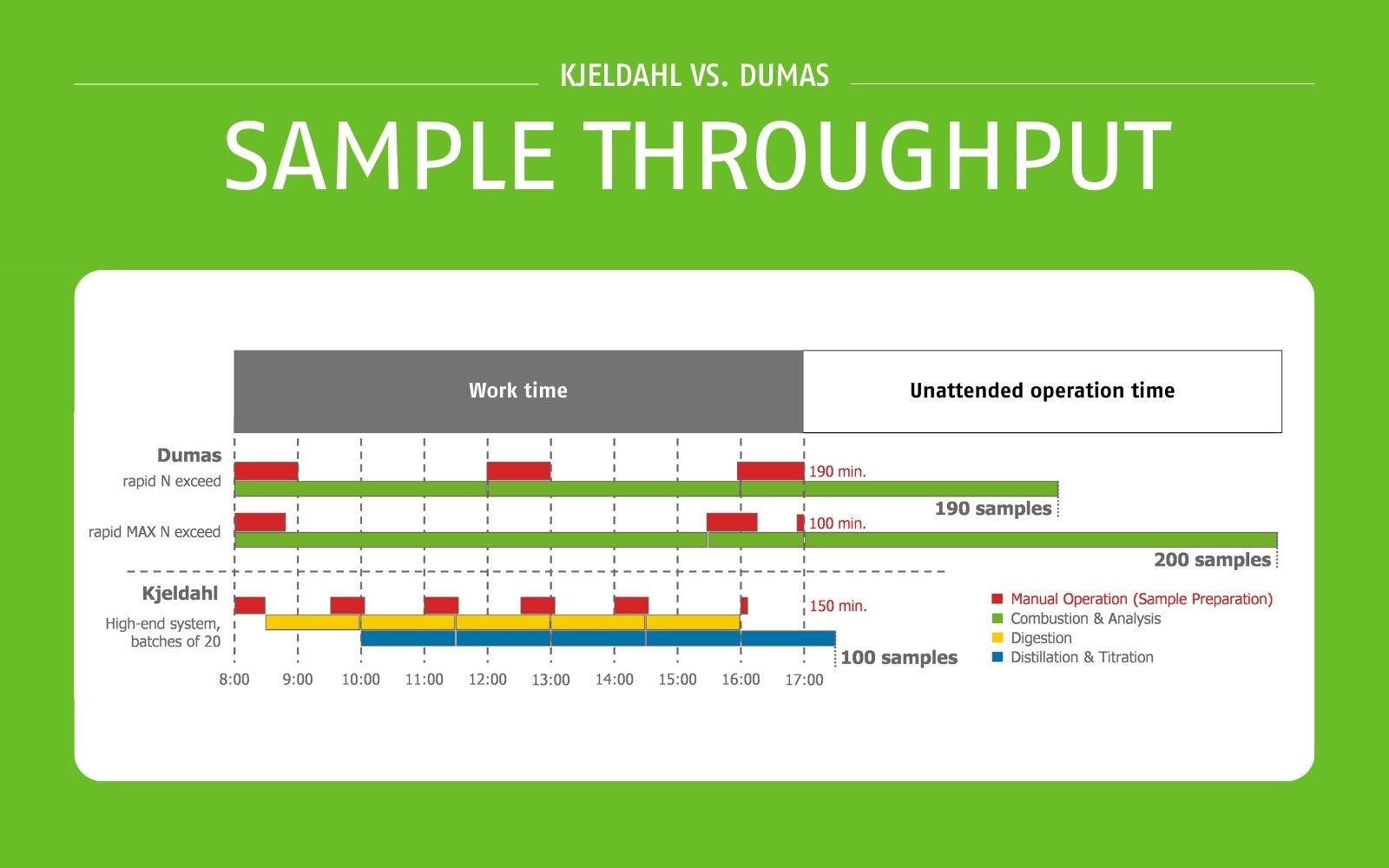
The analysis time for the Kjeldahl method is a minimum of 100 minutes. Using the batch method, a maximum of 100 samples can be analyzed in a day, but a single sample takes the same time as analyzing the entire batch. The method is time-consuming and labor-intensive due to the many manual steps involved in sample preparation and processing.
With the Dumas method of nitrogen determination, the analysis time per sample is four to five minutes, i.e. results (even for individual samples) are available more quickly than with the Kjeldahl method. This makes the Dumas method much more suitable for monitoring and optimizing processes and making timely decisions. In a normal working day, up to 200 samples can be measured with the Dumas method - twice the number of samples as with the Kjeldahl method (see Figure 1). Due to the high degree of automation, samples can be run unattended after weighing: up to seven hours of unattended operation is possible, allowing the operator to focus on other tasks in the laboratory.
Cost per analysis
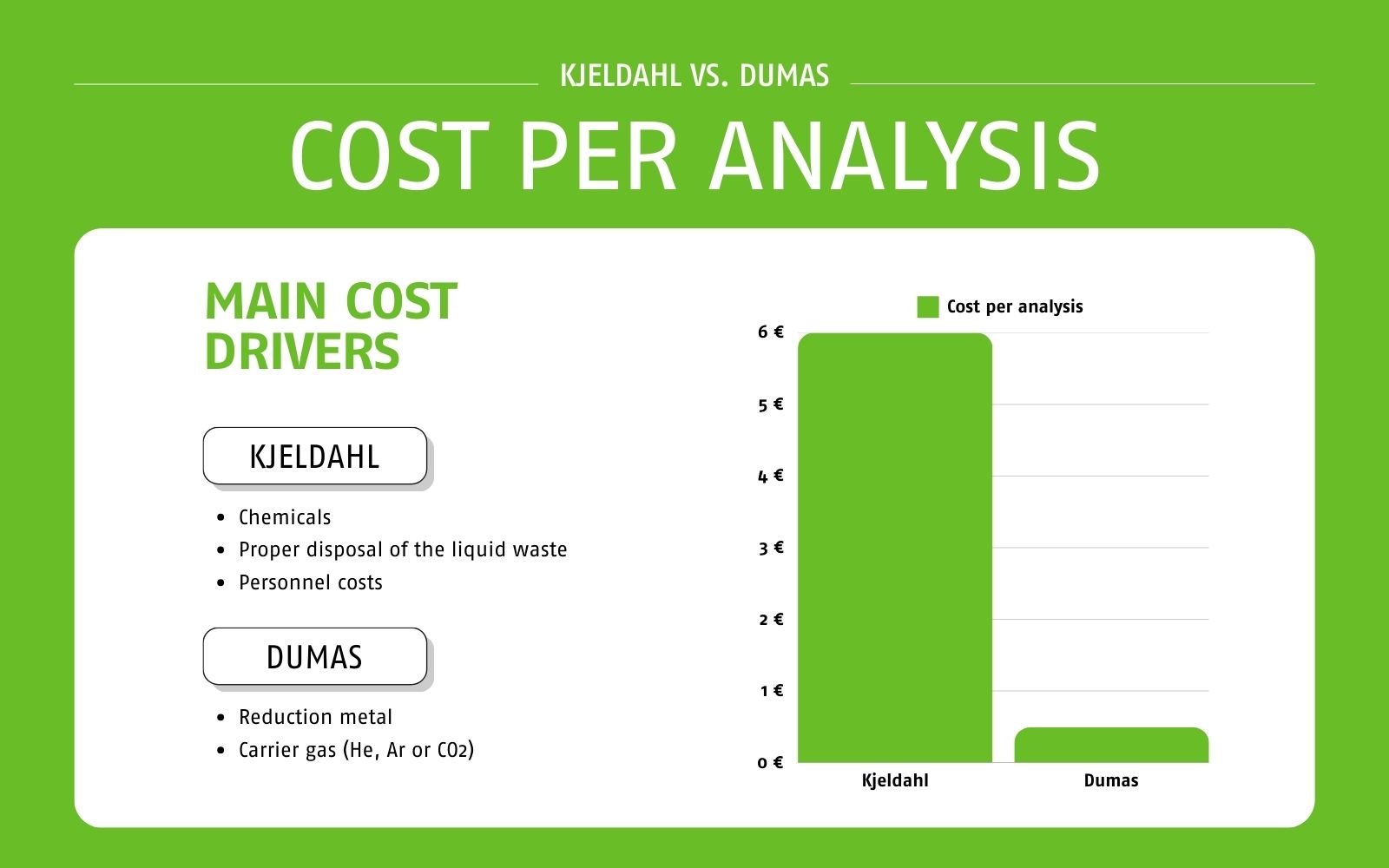
The cost per analysis for the Kjeldahl method is approximately €6*. The main cost factors are the cost of chemicals, their proper disposal and personnel costs. In addition, it requires fume cupboard space, which is usually limited and expensive. In comparison, the cost per analysis with the Dumas method is between €0.25* and €0.49*, depending on the analyzer and the carrier gas used (helium, argon or CO2, depending on instrument model).
* Costs may vary by country
Safety & environmental friendliness
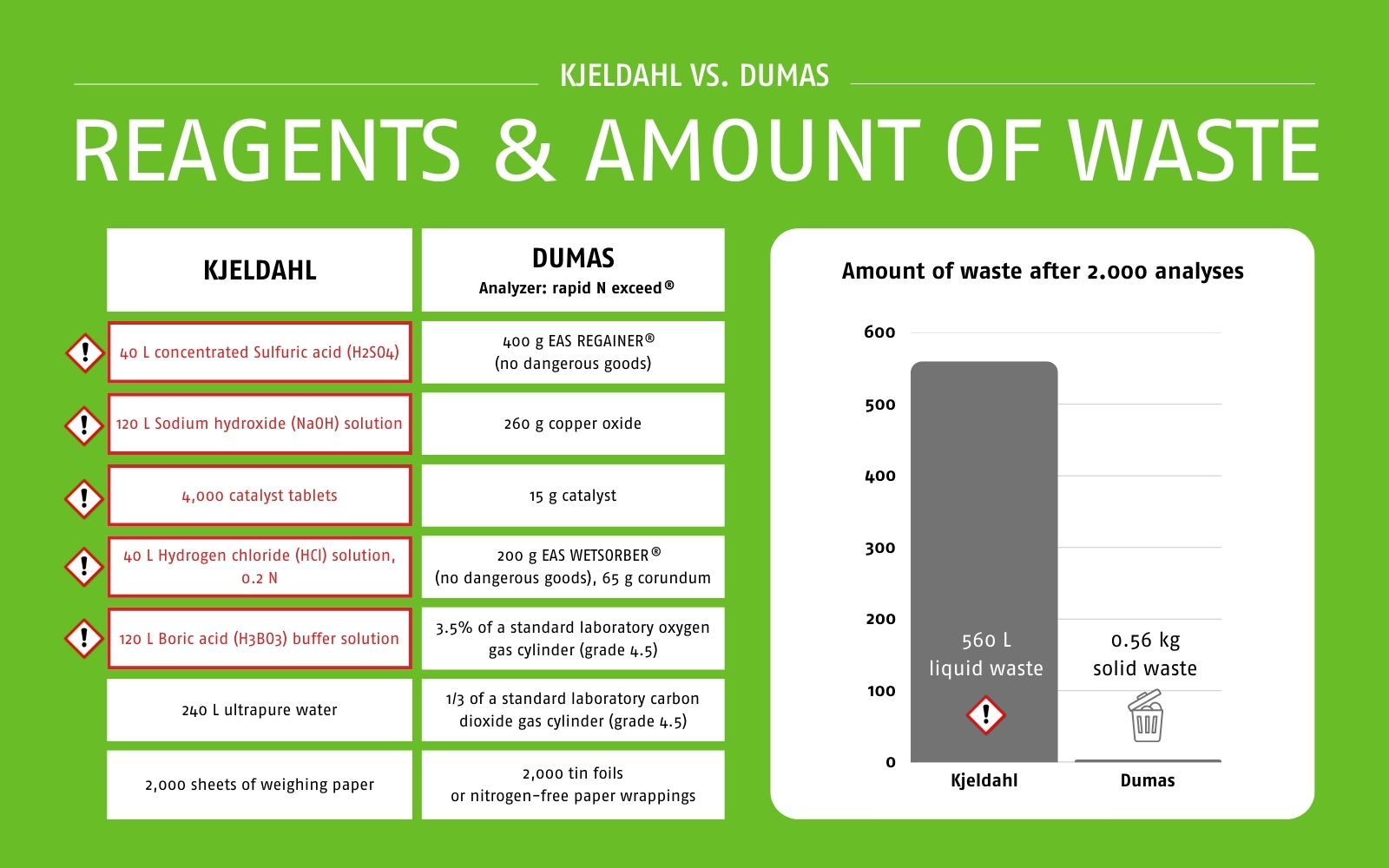
Nitrogen determination according to Kjeldahl uses concentrated sulfuric acid and catalysts that are hazardous to the user and the environment. The analysis process produces harmful fumes that, if not properly extracted, pose a risk to people. The handling of hazardous substances in the workplace also poses a risk. In addition, the liquid waste from the analysis process is harmful to the environment and requires costly disposal. For every 2,000 samples analyzed, approximately 560 liters of chemical waste are generated.
The Dumas method, on the other hand, requires no harmful or toxic chemicals. The analysis process is safe and non-hazardous, and the high degree of automation virtually eliminates operator error. The analysis of 2,000 samples produces only 560 g of waste, which can be disposed of regularly. In direct comparison, the amount of waste generated after 2,000 samples is 1,000 times greater with Kjeldahl than with Dumas (560 liters of liquid waste with Kjeldahl vs. 0.56 kg of solid waste with Dumas using a rapid N exceed® nitrogen/protein analyzer).
Conclusion
Laboratories with a high sample throughput will benefit from the faster Dumas nitrogen determination, as about twice as many samples can be analyzed in the same time as with the Kjeldahl method. When it comes to labor costs, the Dumas method scores because the analysis is largely unattended, freeing lab personnel for other tasks. Due to the higher labor and material costs, as well as the time-consuming disposal of waste, the cost per analysis for Kjeldahl nitrogen determination is significantly higher than for the Dumas method. In addition, companies must ask themselves whether the production of hazardous waste - which could be avoided by using another technology - is still in keeping with the times and their corporate philosophy.
Standards for nitrogen determination according to Dumas
The replacement of the Kjeldahl method by the Dumas method also explains the increasing number of worldwide standards specifying the Dumas method for nitrogen determination. Below you will find an excerpt of Dumas standards, grouped by sample type.
The following standards specify the nitrogen determination according to Dumas for food and beverages (excerpt):
| Standard | Year | Title |
| AOAC 99215 | 1996 | Crude Protein in Meat and Meat Products |
| AOAC 99223 | 1998 | Crude Protein in Cereal Grains and Oilseeds |
| AOAC 99709 | 2008 | Nitrogen in beer, wort and brewing grains. Protein (total) by calculation. Combustion method. |
| ASBC Protein Methods | 2010 | Protein Methods |
| DIN EN ISO 14891 | 2002 | Milk and milk products - Determination of nitrogen content - Routine method using combustion according to the DUMAS principle |
| DIN EN ISO 16634-2 | 2016 | Cereals, pulses, milled cereal products, oilseeds and animal feeding stuffs - Determination of the total nitrogen content by combustion according to the Dumas principle and calculation of the crude protein content |
| EBC | 2016 | Total nitrogen in beer, wort and malt using the Kjeldahl and Dumas method |
| EU Pharm05 TNb | 2005 | Total Protein |
| ICC 167 | 2000 | Determination of crude protein in grain and grain products for food and feed by the Dumas Combustion Principle |
| ISO 14891 | 2002 | Milk and milk products - Determination of nitrogen content - Routine method using combustion according to the Dumas principle |
The following standards specify the nitrogen determination according to Dumas for animal feed (excerpt):
| Standard | Year | Title |
| AOAC 99003 | 1998 | Protein (Crude) in Animal Feed |
| AOCS Ba 4e93 | 1999 | Generic Combustion Method for Determination of Crude Protein |
| DIN EN ISO 16634-1 | 2008 | Food products - Determination of the total nitrogen content by combustion according to the Dumas principle and calculation of the crude protein content - part 1: Oilseeds and animal feeding stuff |
The following standards specify the nitrogen determination according to Dumas for soil samples, plant samples and fertilizers (excerpt):
| Standard | Year | Title |
| AOAC 99313 | 1997 | Nitrogen (Total) in Fertilizers |
| DIN EN 13654-2 | 2002 | Soil improvers and growing media – Determination of nitrogen - Part 2: Dumas method |
| DIN ISO 13878 | 1998 | Soil quality - Determination of total nitrogen content by dry combustion (elemental analysis) |
The following standards specify the nitrogen determination according to Dumas for waste (excerpt):
| Standard | Year | Title |
| DIN EN 16168 | 2016 | Sludge, treated biowaste and soil - Determination of total nitrogen using dry combustion method |
The following standards specify the nitrogen determination according to Dumas for liquid fuels (excerpt):
| Standard | Year | Title |
| ISO 18611-2 | 2014 | Ships and marine technology - Marine NOx reduction agent AUS 40 - Part 2: Test methods |
| ISO 22241-2 | 2019 | Diesel engines — NOx reduction agent AUS 32 |
The following standards specify the nitrogen determination according to Dumas for rubber (excerpt):
| Standard | Year | Title |
| ISO 24698-1 | 2018 | Rubber, raw — Determination of bound acrylonitrile content in acrylonitrilebutadiene rubber (NBR) — Part 1: Combustion (Dumas) method |
Further information
Request access to our on-demand webinar to learn more about differences between the Dumas method and classical protein determination according to Kjeldahl, functionality of nitrogen/protein determination according to Dumas, the range of applications and sample types for nitrogen/protein determination according to Dumas, and decision criteria for laboratories when selecting a suitable nitrogen/protein analyzer.
Language: English | Duration: 28 minutes
Webinar "Fast and precise determination of N-protein using the Dumas combustion"

REQUEST VIDEO
Fill in the form to receive your access link by e-mail.
Your contractual consideration for the free provision of the video is the subscription to our personalized newsletter. By clicking on the “request now” button, you therefore declare your acceptance of the receipt of personalized newsletters by e-mail by Elementar Analysensysteme GmbH and its group companies as well as the evaluation of your user behavior in this regard and - if available - the merging of this data with your data in our customer database.
In order to receive newsletters from our group companies, it is necessary to transfer your above-mentioned personal data to these companies. We point out that these are partly located in so-called unsafe third countries outside the EU/EEA, in which no adequate level of data protection (e.g. by adequacy decision of the EU, Art. 45 GDPR) is guaranteed. In these countries, you may not be able to enforce your rights as a data subject, or only to a limited extent. In addition, it is possible that local government agencies access your data to a disproportionate extent. The data transfer is based on Art. 49 para. 1 lit. b) GDPR.
You are aware that the subscription to our personalized newsletter represents the contractual consideration that you provide for the free provision of the video. You can unsubscribe from the newsletter at any time with effect for the future. You can object to the future use of your data for advertising purposes at any time. For further information, please refer to our privacy policy.
For more information on the difference between the Kjeldahl and Dumas methods, download our Technical Note below.
In our Application Notes, you can find out how the Dumas method and the Kjeldahl method compare in the analysis of milk and dairy products.
Read our Customer Spotlights to learn why laboratories around the world are replacing Kjeldahl analysis with the Dumas combustion method for nitrogen and protein determination.