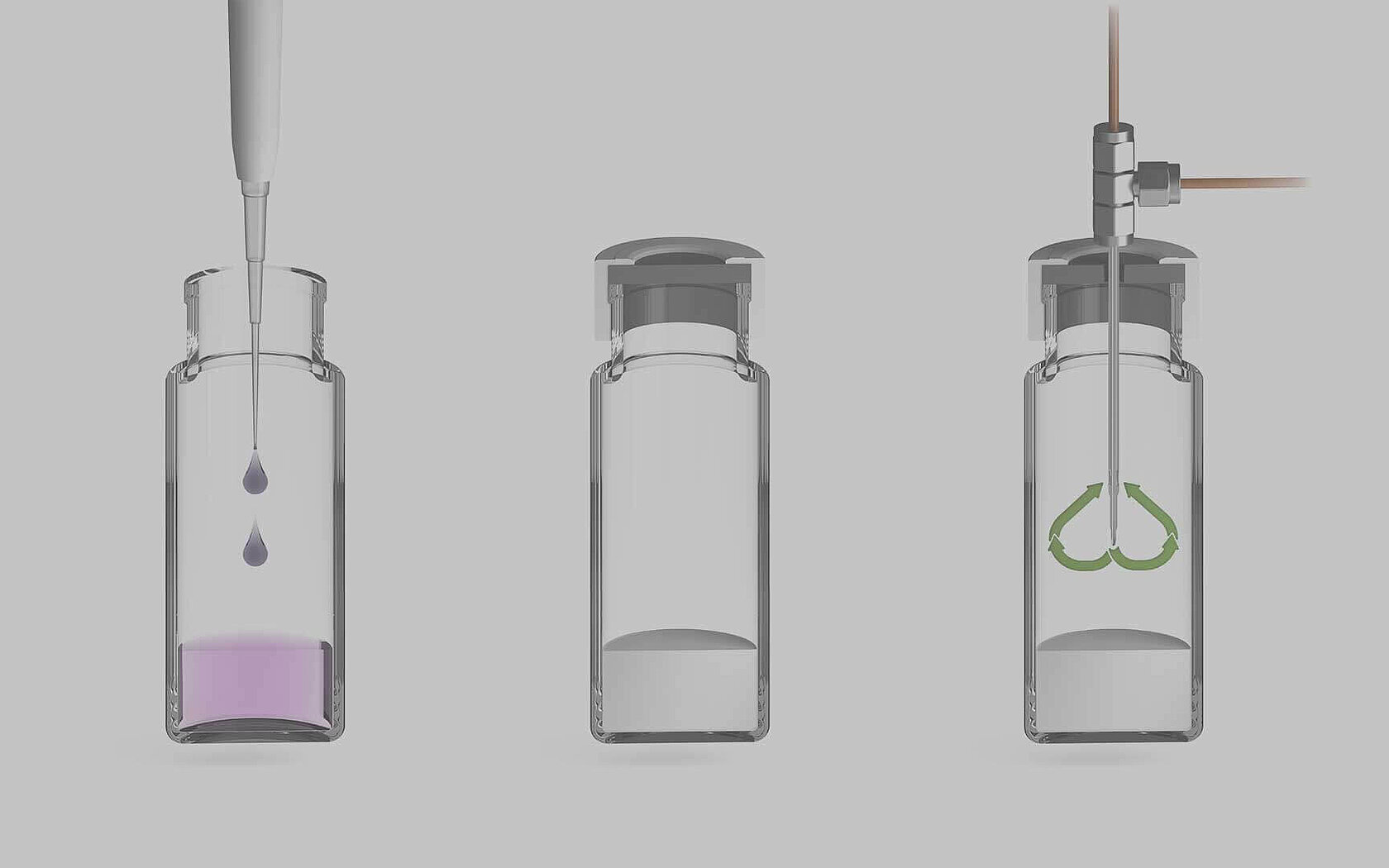Looking for an easier way to analyze 15N and 18O of dissolved nitrate?
Do you spend large amounts of time preparing and growing Pseudomonas aureofaciens? Fed up of completing risk assessments when you are trying to purchase or dispose of dangerous chemicals? Maybe the idea of dealing with these problems has dissuaded you from starting to analyze 15N and 18O of dissolved nitrate altogether? Now you have another option.
Understanding Nitrate Pollution
On-Demand Webinar Series
Elementar is proud to present a series of webinars hosted by academic experts describing the impacts of nitrate pollution and how we can develop solutions to reduce nitrate in the environment. Critical to developing these solutions is stable isotope analysis, an analytical technique which can inform scientists about the possible sources of pollution.

Titanium (III) reduction of nitrate is quick and safe
A groundbreaking new paper from Altabet et al.* has described a new technique for the conversion of dissolved nitrate to N2O without using hazardous reagents or bacterial cultures giving fully prepared samples within 24 hours. With easily obtained reagents and basic laboratory infrastructure, novice labs can perform high quality isotopic analysis of nitrate.
* Altabet, M.A., Wassenaar, L.I., Douence, C., Roy, R. (2019) A Ti(III) reduction method for one-step conversion of seawater and freshwater nitrate into N2O for stable isotopic analysis of 15N/14N, 18O/16O and 17O/16O, Rapid Communications in Mass Spectrometry, Vol 33, Issue 15, Pages 1227-1239. Link: https://doi.org/10.1002/rcm.8454
Hear from the method's co-creator in our whitepaper
Explore Dr. Wassenaar’s new method and learn what it could mean for the future of nitrate isotope analysis

DOWNLOAD YOUR COPY
Fill in the form to receive your download link per e-mail.
Your contractual consideration for the free provision of the download is the subscription to our personalized newsletter. By clicking on the “download now” button, you therefore declare your acceptance of the receipt of personalized newsletters by e-mail by Elementar Analysensysteme GmbH and its group companiesas well as the evaluation of your user behavior in this regard and - if available - the merging of this data with your data in our customer database.
In order to receive newsletters from our group companies, it is necessary to transfer your above-mentioned personal data to these companies. We point out that these are partly located in so-called unsafe third countries outside the EU/EEA, in which no adequate level of data protection (e.g. by adequacy decision of the EU, Art. 45 GDPR) is guaranteed. In these countries, you may not be able to enforce your rights as a data subject, or only to a limited extent. In addition, it is possible that local government agencies access your data to a disproportionate extent. The data transfer is based on Art. 49 para. 1 lit. b) GDPR.
You are aware that the subscription to our personalized newsletter represents the contractual consideration that you provide for the free provision of the download. You can unsubscribe from the newsletter at any time with effect for the future. You can object to the future use of your data for advertising purposes at any time. For further information, please refer to our privacy policy.
Celebrating 25 Years of Dr Carol Kendall’s Dual Isotope Plot of Nitrate Sources
An interview with Dr Kendal
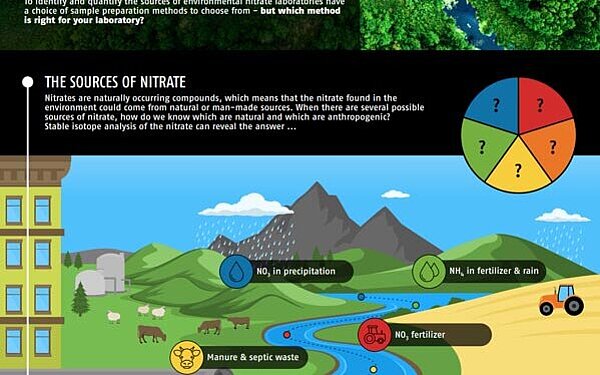
Stable isotope analysis enables researchers to identify the likely dominant sources of nitrate, whether from agricultural fertilisers, animal manure, improperly treated sewage and wastewater, or other sources. The dual isotope plot of nitrate sources makes it simple to visualise the likely sources of nitrate in water samples, as the first step towards implementing solutions.
We sat down with Carol to discuss how she developed the now-famous dual isotope plot, the impact of her work over the last quarter-century, and the applications of her research to address urgent concerns about environmental pollution.

Choosing the right method for your nitrate isotope analysis
Nitrogen pollution is one of the world's key environmental challenges, posing threats to water quality and accelerating climate change. Through overuse of synthetic fertilizers, increasing numbers of livestock and the burning of fossil fuels, the amount of nitrate entering the environment is unsustainable. To identify and quantify the sources of environmental nitrate laboratories have a choice of sample preparation mehods to choose from - but which method is right for your laboratory?
Simple, one-step reaction
1. Add reagents and sample to 40 ml or 20 ml vial using pipette
2. Leave vials 12—24 hrs to react (conversion of sample nitrate to N2O)
3. Run samples on IRMS system
Learn more about this method of sample preparation by downloading our whitepaper.
Already established your nitrate method?
In 2001, Sigman & Casciotti published their breakthrough paper which described using denitrifying bacterial cultures to convert nitrate to N2O for 15N analysis. Then a year later, the method was updated to perform dual isotope 15N and 18O analysis from a single freshwater or seawater sample, allowing sources of nitrate to be easily resolved. This method, and the cadmium-azide method (Altabet et al., 2005) have been widely adopted by laboratories around the world. Our stable isotope analyzer EnvirovisION is fully compatible with these methods making it ideal for existing laboratories which use these techniques.
Watch Dr Andi Smith's full lecture from the Elementar UK IRMS User Meeting at this link.
Introducing EnvirovisION
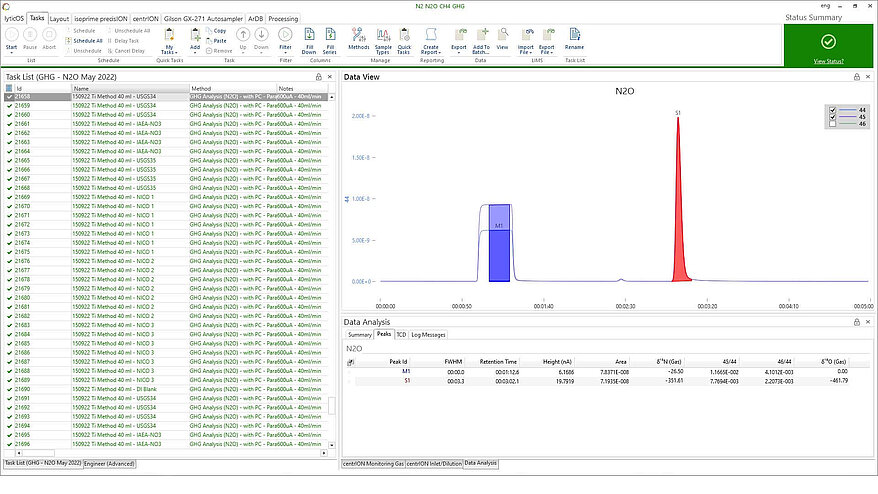
Stable isotope analyzer for the isotopic analysis of dissolved nitrate
EnvirovisION is a specially configured stable isotope analyzer for the isotopic analysis of dissolved nitrate samples and has been enhanced to utilize the new titanium (III) reduction* sample preparation method. The system has numerous features designed to automate, simplify, and improve sample throughput allowing you to generate more data, faster.
Simpler, faster dissolved nitrate analysis with EnvirovisION
The ideal solution for quantifying the global nitrogen cycle
Downloads



Stay up-to-date
✉︎ NEWSLETTER
Stay up-to-date and register for our newsletter to receive latest news and publications on stable isotope analysis.
Balancing the nitrogen cycle
All life depends on the availability of nitrogen. It is an essential nutrient yet is almost completely inaccessible in its most abundant form, N2. Elemental nitrogen is therefore consumed and expelled via myriad chemical, physical and biological processes which perpetuate the global nitrogen cycle, holding natures balance in check. But for the last 100 years, anthropogenic activity has tipped these balances in ways we do not yet fully understand. The price of being able to feed the global population and provide them with energy may prove high.
How the shifts and balances in the nitrogen cycle ultimately play out due to anthropogenic inputs is unknowable, but we must attempt to understand this intricate system if we are to mitigate the worst effects of our inputs into the nitrogen cycle. Stable isotope analysis is an essential tool as we attempt to do that.
Contact us
Do you have questions or challenges and need an expert to provide straightforward support? You are evaluating different methods and need our help? Our experts are available to answer all your questions about isotopic analysis of dissolved nitrate samples.
If you would like to receive our newsletter, by clicking on the “Submit” button you consent to receiving personalized newsletters by email from Elementar Analysensysteme GmbH and its group companies, as well as to the evaluation of my user behavior in this regard and – if available – the merging of this data with my data in our customer database.
In order to receive newsletters from our group companies, it is necessary to transmit the above-mentioned data to them. We would like to point out that some of our group companies are located in so-called unsafe third countries outside the EU/EEA, where an adequate level of data protection (e.g. through an adequacy decision by the EU as defined in Art. 45 GDPR) is not guaranteed. There, you may not be able to enforce your rights as a data subject, or only to a limited extent. In addition, it is possible that state agencies there may access your data to an unreasonable extent. The data transfer to these recipients is therefore only legitimized by your consent according to Art. 49 para. 1 lit. a) GDPR, which you give by clicking on the “Submit” button.
The newsletter can be canceled at any time with effect for the future and my consent to the third-country transfer can also be revoked at any time. A revocation does not affect the legality of the processing carried out on the basis of the consent until the revocation. For more information, please refer to our privacy policy
Would you like to receive the latest information on our solutions for stable isotope analysis?
Subscribe to our newsletter and be always up-to-date.